January 22, 2025
Johnson & Johnson JNJ announced that the FDA has approved its supplemental new drug application (sNDA), seeking approval for Spravato (esketamine) as a monotherapy for adults living with treatment-resistant depression (TRD).
Spravato is presently approved in combination with an oral antidepressant to treat adults with TRD and depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior.
Following the latest nod, Spravato became the first and only monotherapy for treating MDD in adults who have had an inadequate response to at least two oral antidepressants and are considered to have TRD.
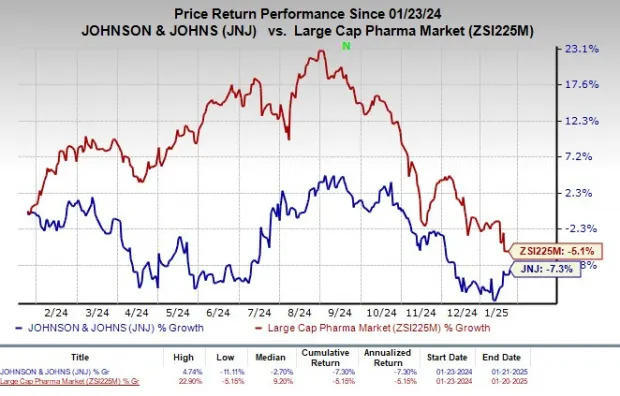
In the past year, shares of J&J have lost 7.3% compared with the industry’s decline of 5.1%.

The latest FDA nod for Spravato was based on data from a double-blind, multicenter, placebo-controlled study.
Data from the study showed that treatment with Spravato alone led to a rapid and superior improvement in the Montgomery-Asberg Depression Rating Scale (MADRS) total score compared to placebo.
Also, treatment with Spravato alone demonstrated a rapid and superior improvement in depressive symptoms versus placebo as early as 24 hours at 4 weeks – meeting the study’s primary endpoint.
Importantly, the safety profile of Spravato monotherapy was similar to the existing clinical and real-world data when used in combination with an oral antidepressant.
Per the company, with this approval for Spravato as a standalone treatment, patients might experience improvements in their depressive symptoms as early as 24 hours and at 28 days without the need for daily oral antidepressants.
In the first nine months of 2024, Spravato recorded sales worth $780 million, up 61.5% year over year, driven by the ongoing launch and increased physician and patient demand.
Approval for expanded indications should boost the drug’s sales in future quarters.
J&J currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are BioMarin Pharmaceutical Inc. BMRN, Voyager Therapeutics, Inc. VYGR and Castle Biosciences, Inc. CSTL, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here .
In the past 60 days, estimates for BioMarin’s earnings per share have increased from $3.94 to $4.02 for 2025. In the past year, shares of BMRN have plunged 32.8%.
BMRN’s earnings beat estimates in each of the trailing four quarters, the average surprise being 28.70%.
In the past 60 days, estimates for Voyager Therapeutics’ loss per share have narrowed from $1.72 to $1.48 for 2025. In the past year, shares of VYGR have plunged 30.9%.
VYGR’s earnings beat estimates in each of the trailing four quarters, the average surprise being 120.87%.
In the past 60 days, estimates for Castle Biosciences’ loss per share have narrowed from $1.88 to $1.84 for 2025. In the past year, shares of CSTL have surged 35.5%.
CSTL’s earnings beat estimates in each of the trailing four quarters, the average surprise being 172.72%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
BioMarin Pharmaceutical Inc. (BMRN) : Free Stock Analysis Report
Voyager Therapeutics, Inc. (VYGR) : Free Stock Analysis Report
Castle Biosciences, Inc. (CSTL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research