January 16, 2025
Johnson & Johnson JNJ has initiated a rolling submission with the FDA seeking approval for TAR-200, its investigational drug-device combination to treat certain patients with non-muscle invasive bladder cancer (NMIBC).
The J&J filing seeks the FDA’s nod for TAR-200 as a single agent to treat patients with BCG-unresponsive, high-risk NMIBC with carcinoma in-situ (CIS), with or without papillary tumors. If approved, TAR-200 will be the first intravesical drug-releasing system in the given indication.
The FDA will review this submission under its Real-Time Oncology Review (“RTOR”) program.
Filings reviewed under the RTOR program enable the agency to start evaluating portions of the application as soon as they are ready, instead of waiting for the entire application to be submitted. This approach has been designed to accelerate the approval process for certain oncology drugs or biologics that aim to address unmet medical needs or provide significant advantages over existing therapies.
TAR-200 is an innovative method developed by J&J for a sustained delivery of chemotherapy drug gemcitabine directly into the bladder. Following a prospective FDA nod, J&J aims to offer a potentially less invasive alternative to the current standard treatment, radical cystectomy (complete bladder removal).
The RTOR program also works alongside the FDA’s other programs to expedite drug development and review. J&J’s TAR-200 has been granted breakthrough therapy designation by the FDA in the given indication.
The FDA filing is supported by data from the phase IIb SunRISe-1 study, which revealed that treatment with J&J’s drug-device combo achieved high effectiveness and durability with an 83.5% complete response (CR) rate without the need for reinduction. Per management, 82% of responders maintained the CR at a median follow-up of nine months.
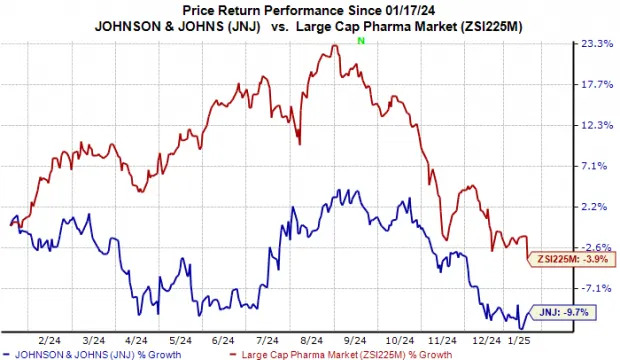
In the past year, J&J’s shares have lost nearly 10% compared with the industry’s 4% decline.

TAR-200 provides a novel treatment approach for an indication with limited therapeutic options, especially for older patients unable or unwilling to undergo radical cystectomy.
If approved, J&J’s TAR-200 will likely hold an edge over ImmunityBio ’s IBRX Anktiva and Merck ’s MRK Keytruda, both of which are approved to treat high-risk NMIBC in patients unresponsive to BCG vaccine. However, the Merck and ImmunityBio drugs utilize a different mechanism of action — IBRX’s Anktiva is an IL-15 antibody drug, approved for intravesical use in combination with the BCG vaccine. MRK’s Keytruda is a blockbuster PD-1 inhibitor approved for use as a single agent. However, it is administered intravenously.
Although primarily used to prevent tuberculosis, the BCG vaccine is also a standard treatment for some forms of bladder cancer.
Johnson & Johnson price | Johnson & Johnson Quote
J&J currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here .
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
ImmunityBio, Inc. (IBRX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research