January 10, 2025


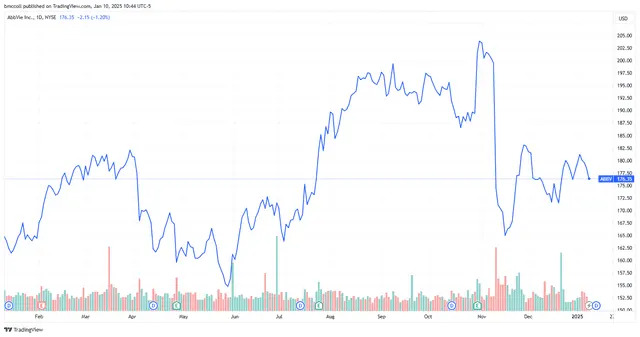
AbbVie ( ABBV ) shares fell Friday after the biotech firm announced it would take an estimated $3.5 billion impairment charge because of the failure of one of its drugs.
The company wrote in a regulatory filing that the charge was related to emraclidine, an experimental medicine to treat schizophrenia in adults that AbbVie acquired when it purchased Cerevel Therapeutics Holdings.
AbbVie reported in November that a Phase 2 trial of emraclidine showed the treatment did not meet primary endpoints.
In the filing, the company explained that following the study, it began an evaluation of the impact of the results, "which resulted in a significant decrease in the estimated future cash flows for the product."
Shares of AbbVie, which fell 1% in recent trading, are up about 7% over the past year.
Read the original article on Investopedia