January 8, 2025
For Geron Corporation GERN, 2024 was a transformational year as it saw the FDA approval and commercial launch of Rytelo (imetelstat) for the treatment of low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia in June.
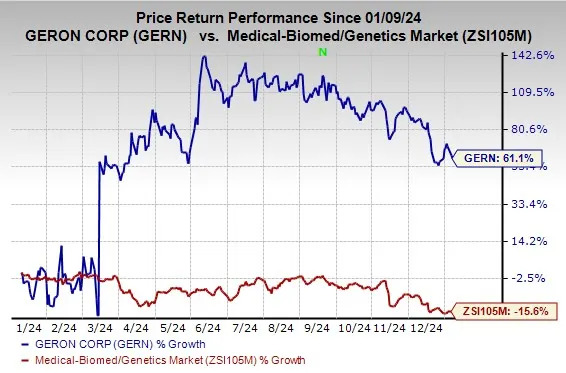
Geron’s shares have risen 61.1% in the past year against a decrease of 15.6% for the industry. Here we discuss some reasons for the same.

The approval of Rytelo gave Geron its first FDA-approved product. In its first full quarter since the U.S. launch, Rytelo recorded sales of $28.2 million (in the third quarter of 2024), which exceeded the company’s expectations. Following high unmet need in lower-risk MDS, Geron is confident of seeing continued demand and momentum for Rytelo.
In December, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency gave a positive opinion recommending the approval of Rytelo in the EU. The European Commission is expected to give its decision regarding the approval of Rytelo in the first half of 2025.
Geron is also evaluating Rytelo in the phase III IMpactMF study in patients with relapsed/refractory myelofibrosis (“MF”). Early findings from the IMproveMF study support the potential tolerability of imetelstat and ruxolitinib as a combination therapy for the treatment of MF.
Geron also completed important synthetic royalty and debt financing transactions with Royalty Pharma and Pharmakon Advisors, which strengthened the company’s cash position. The synthetic royalty agreement with Royalty Pharma provides it with $125 million of capital in exchange for tiered royalty payments. Investment funds managed by Pharmakon Advisors, LP have committed to a 5-year, senior secured term loan of up to $250 million. The funding agreement strengthens the company’s balance sheet to support the commercial launch of Rytelo in the United States and potential launch in the EU, as well as the ongoing study in MF and other uses.
In the past 60 days, 2024 loss estimates for Geron have improved from 26 cents per share to 25 cents per share. For 2025, loss estimates have improved from 10 cents to 6 cents per share over the same timeframe.
Geron has a Zacks Rank #3 (Hold).
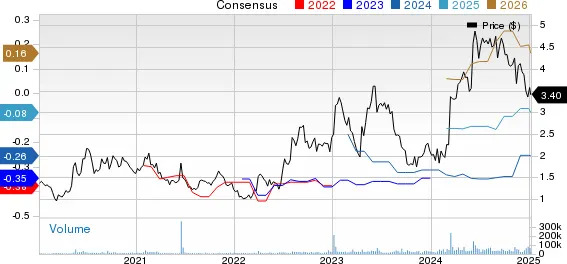
Geron Corporation price-consensus-chart | Geron Corporation Quote
Some top-ranked stocks from the biotech sector are Puma Biotechnology PBYI Halozyme Therapeutics HALO and Castle Biosciences CSTL, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for Halozyme Therapeutics’ 2025 earnings per share have increased from $4.80 to $4.81. In the past year, shares of HALO have risen 44.2%.
HALO’s earnings beat estimates in three of the trailing four quarters while meeting the same on the remaining occasion, the average surprise being 14.86%.
In the past 60 days, estimates for Castle Biosciences’ 2025 bottom line have narrowed from a loss of $1.88 per share to a loss of $1.84 per share. In the past year, shares of CSTL have surged 47.1%.
CSTL’s earnings beat estimates in each of the trailing four quarters, the average surprise being 172.72%.
In the past 60 days, estimates for Puma Biotechnology’s 2025 earnings per share have increased from 42 cents to 54 cents. In the past year, shares of PBYI have declined 22.4%.
PBYI’s earnings beat estimates in three of the trailing four quarters while missing the same on the remaining occasion, the average surprise being 32.78%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Geron Corporation (GERN) : Free Stock Analysis Report
Halozyme Therapeutics, Inc. (HALO) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Castle Biosciences, Inc. (CSTL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research